Just How the Heck Did the Original Voltaic Pile Work?

He didn't invent it.
Today virtually all the appliances that plug into a wall outlet use alternating current. That, is the electrons don't travel down the wire but instead just vibrate back and forth.
But the first commercial electricity was direct current. That's because the earliest electricity was generated by batteries. This early electricity mostly went to power the telegraph. Telegraphs don't need a lot of energy, so battery power was enough.

Nikola Tesla
He didn't invent it either.
But if you do need lots of electricity it's really better to use generators which are cheaper than batteries and can draw on other sources of power - steam, water, wind - to make the electricity. But electrical generators normally create alternating current. So the early generators had a part called a commutator that stopped the current from reversing. These generators produced a pulsating direct current but it was in one direction. So you could at least use direct current devices with this generator generated electricity.
But a constant unwavering current is easiest to produce from batteries, And with the advent of the electronic age, DC current has seen a resurgence and more and more AC current is being converted to DC to recharge our batteries.
Although you'll read that batteries may have been invented in antiquity, the first clear-cut case of making a battery was by the Italian inventor, Alessandro Volta. That was in 1800.
What Alessandro did to sandwich salt-water soaked bits of paper or felt between disks of zinc and copper. If you then hook each end to a wire, you get an electrical circuit.
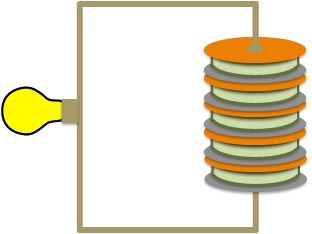
Voltaic Pile
These "voltaic piles" (as we call them today could) create a continuous source of direct current. By stacking more and more of the disc-and-paper sections on top of each other, you increase the voltage. Practical concerns of construction limit the piles to producing maybe a couple of volts, but you could make a bunch of them and hook them up together "in series". You then had a big battery capable of generating so much voltage and current that it could be dangerous.
Despite the simple construction of the voltaic pile, how these suckers work often confounds students. The answers tend to mention simply that the electromotive force is due to the zinc and copper half-reactions:
Zn(s) → Zn+2(aq) + 2e-
Cu+2(aq) + 2e- → Cu(s)
... which produces the overall reaction:
Zn(s) + Cu+2(aq) → Zn+2(aq) + Cu(s)
So we see the neutral zinc atoms gives up two electrons to a positively charged copper ion to form a positive zinc ion and a neutral copper atom.
Without going into the details of the calculations, you can show that the Standard Gibbs Free Energy of the reaction is negative:
ΔGO = -55 Food Calories
... and a negative value means the reaction proceeds spontaneously. So our reaction moves forward, the electrons get shifted from atom to atom, and when they do so they end up going to the wires and move through the circuit. Hey, presto!, we have a battery.
But wait!, the students say. Alessandro had zinc and copper disks and the solution in the filter papers was brine. That is, sodium chloride, just regular table salt made up of sodium and chlorine ions. So where the heck do the zinc and copper ions - that is Zn+2 and Cu+2 - come from?
A search on the Fount of All Knowledge shows this question has been asked but the answers oft times avoid unambiguous resolution. Usually the batteries are shown with copper sulfate as the electrolyte by the copper and zinc sulfate as the electrolyte for the zinc. The two cells are connected by a salt bridge or porous membrane and so we have a circuit for the electrons to flow.
But that's not the answer to our question.
What we're asking is how does sandwiching paper saturated with table salt between zinc and copper disks create electricity? We'd really like to know this.
I thought you would, as Captain Mephisto said to Sidney Brand. It's very simple, really.
First, what we'll do is look at what happens when we make the pile. Or rather, we'll make one cell of the pile.
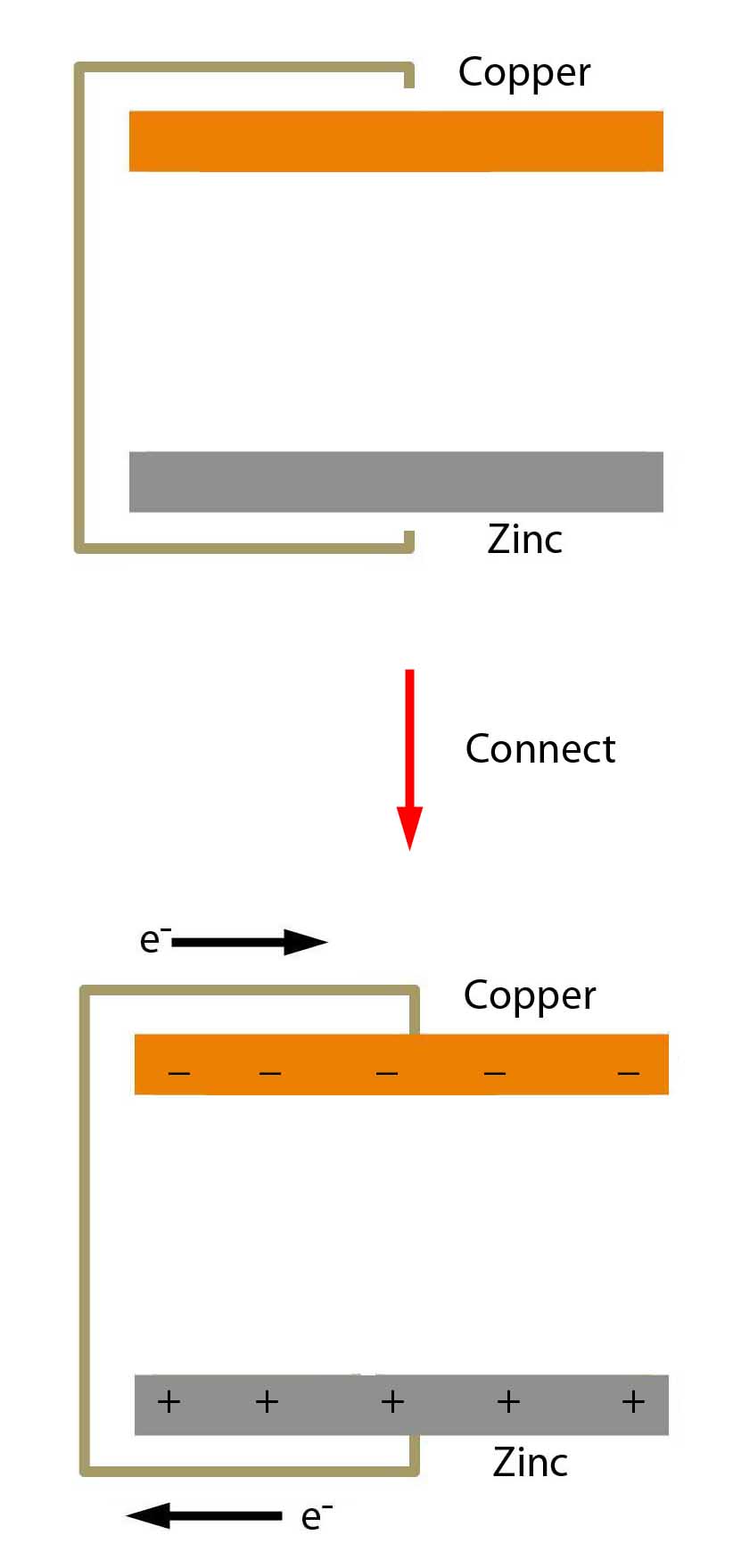
Dissimilar Metals
OK. We take a disk of copper and a disk of zinc and a piece of wire. But the wire isn't yet hooked up. Obviously no electrons can flow through the wire.
But once we do hook the wire up, we find that electrons flow from the zinc to the copper. This is just a natural phenomenon because copper has a greater affinity for electrons than zinc. And metals - like zinc and copper - have "mobile" electrons that like to move from atom to atom.
But ....
Once the wire is hooked up, the flow of electrons would quickly stop. That's because once electrons begin to build up in the copper, the accumulated negative charge begins to repel other incoming electrons. And so the flow of electrons stops.
Nonetheless, the small charge difference does set up an electric field between the two metal disks. Conventionally the field is usually represented as the way a positive charge would move (that is, from the zinc to the copper).
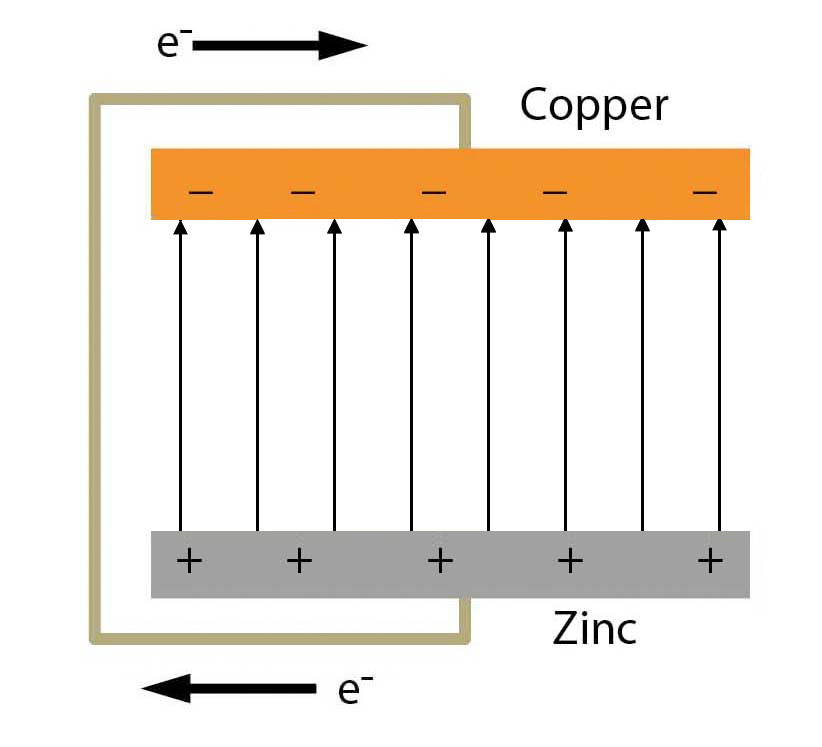
Dissimilar Metals - The Electric Field
If you now put a negatively charged particle between the disks, the particle would move away from the copper - with its excess negative charge - and toward the zinc - with its positive charge. A positively charged particle would move away from the zinc and toward the copper. The ability to move the particles means we have an electric potential difference between the copper and zinc.
At this point we will have a slight digression. Those who are familiar with electricity will say, hold on, there, Pilgrim. The copper in a battery is the cathode of a battery and is positive. The zinc is the anode and is negative. And the whole world knows that current flows from the cathode to the anode - from positive to negative.
Explain that!
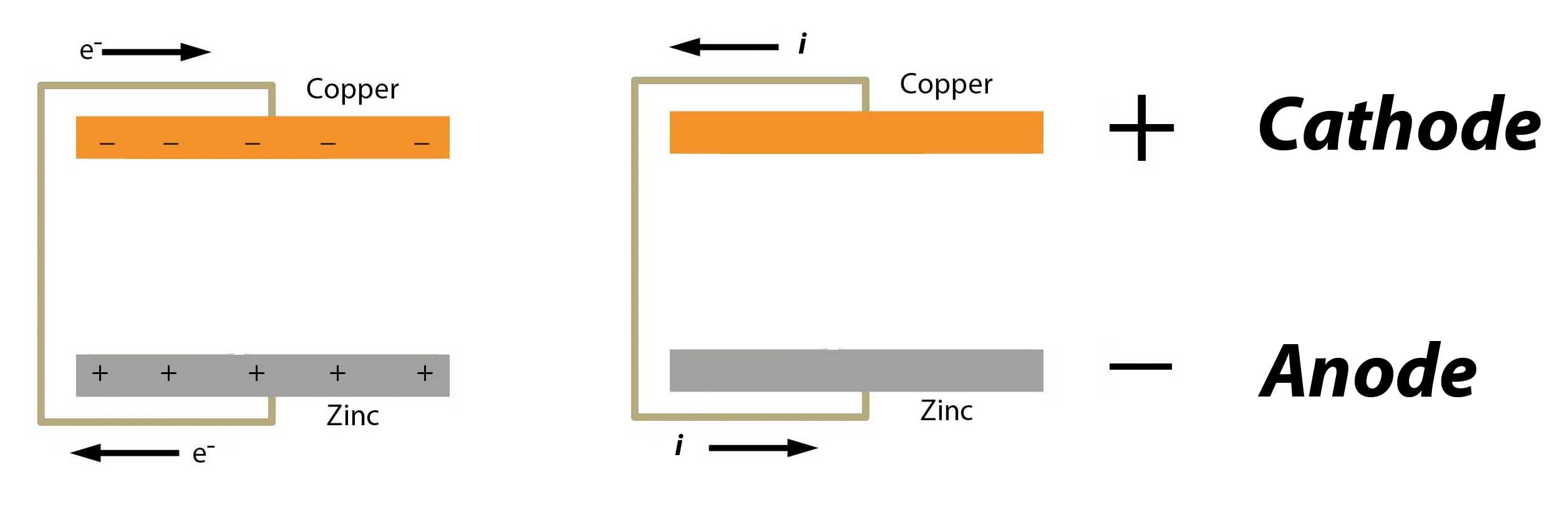
Current Flow
Reality vs. Convention
We can do so quite easily. And it's all because of Benjamin Franklin.
When Benjamin first published his theory of electricity he didn't have the concept of negatively and positively charged particles. He believed that there was a transfer of an electrical fluid from one object to another. In other words, Ben thought electricity was like pouring water from one glass to another. If you pour water into a container, you can say you've "charged" the glass with the water.
According to Ben, then, if something had too much of the fluid it had a positive charge of the fluid. If there was a deficit of fluid, the "charge" of the fluid was negative. The fluid - the electricity - runs as a "current" from a region of positive charge to negative charge.
Of course, Ben was sort of correct. Electrical current is created by the movement of electrons which is the modern version of Ben's "electric fluid". The movement of electrons from one place to another - the "current" - produces the effects of electricity - either a static spark or a current in a wire.
But Ben was not completely correct. He thought that when he rubbed a glass rod with a silk handkerchief the electric fluid went from the handkerchief to the glass. And so the spark came from the excess "fluid" in the glass rod flowing back to a place with less of the fluid.
Benjamin had a fifty-fifty chance of being correct as to what held more fluid and which direction the "fluid" moved. And he guessed wrong. The glass rod looses electrons when rubbed with a silk handkerchief.
So why didn't scientists make the correction when they found out the truth?
For one thing, the knowledge of what was really going on lagged far behind the engineering. Ben wrote about electricity in 1750. But the electron wasn't even discovered until 1897. That an atom was composed of electrons surrounding a positive nucleus was discovered only in the early 1900's.
So for over a century, people had been following Ben's conventions. You can still use the math, diagrams, and even the symbols from the 19th century for the complex microcircuits we use in today's electronics. The physical devices have changed, yes, but there was no reason to change the conventions originated by Ben.
Today there are some texts that try to bring the engineering in sync with the science and talk about the current flowing the same direction as the electrons. But most textbooks still talk of "conventional" current moving opposite the flow of electrons. And so today we still talk of current flow from the positive electrodes to the negative.
After all, then, in many ways we can just say that Benjamin was right. We said the positive charge moved the opposite of the way the negative electrons flow - we didn't say the atoms had to move. And to see how positive charges move to the right even though they don't move, just click the image on the right.
Now back to the workings of the voltaic pile.
We'll continue by adding the sodium chloride and water between our metal disks. And we'll stick a light bulb in the circuit.
Remember some points:
 |
If you change the electrolyte you change the chemistry. |
 |
The electrolytes don't need to have the same elements as the electrodes. |
This last point is important. If the electrodes and the electrolytes have the same metals, it is easier to understand the electrochemical "half-reactions" you see in chemistry textbooks. But you don't need to have the same elements in the electrolytes and in solution as long as they will produce a reaction.
There are two other points that is often obscured in the plethora of diagrams and equations:
 |
The electrolytes provide a source of electrons. |
 |
The electrolytes take in electrons. |
... and this means that ...
 |
An individual electron doesn't need to make it all the way around the circuit to have current in the wire. |
And the figure at right gives a succinct picture of what goes on in a cell of the old style voltaic pile. That is zinc and copper discs separated by paper or felt soaked in brine.
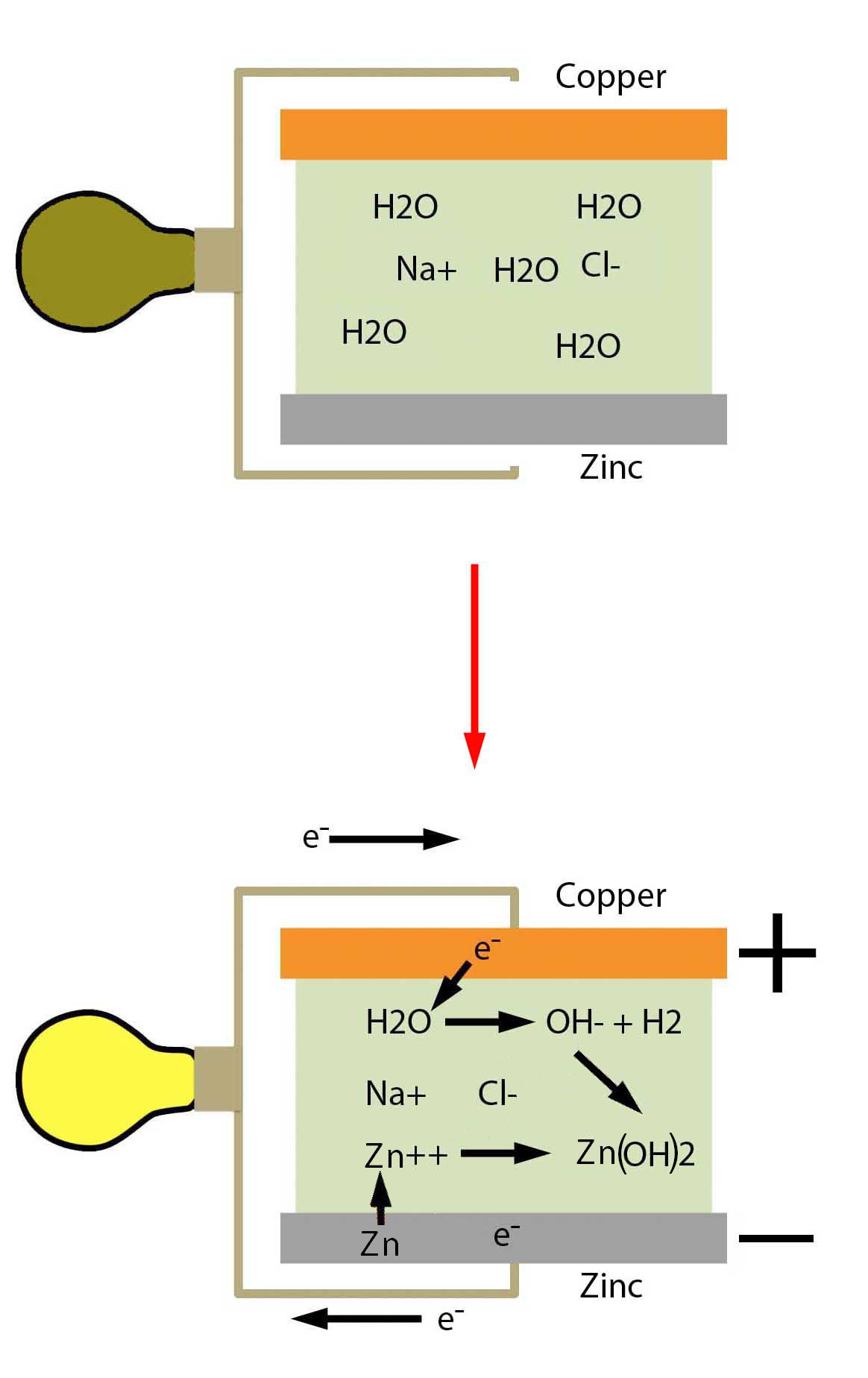
The Voltaic Pile
What Goes On
If at this point you can't say "I understand", we'll add a little elaboration.
OK. We have a voltaic cell, and the zinc and copper electrodes are connected. We have table salt dissolved in water sandwiched between the disks.
Since the table salt - sodium chloride - is dissolved in water, the sodium and chlorine are free to move around as ions. The sodium has a +1 charge and the chlorine a -1 charge.
Remember that we have the electrons move from the zinc down the wire to the copper. The driving force is the fact that the two dissimilar metals have different affinities for electrons. The copper wants the electron and the zinc is willing to give them up.
But that leaves a zinc atom without its full complement of electrons. Zinc prefers to give up two electrons rather than one and so we end up with the Zn+2 cation.
Zn0 (metal) → Zn+2 + 2e- (to the wire)
And the charge of the zinc has to be balanced out. It's tempting to take two chloride anions that were from the sodium chloride solution.
Zn0 (metal) → Zn+2 + 2Cl+1
But what about the sodium ions? You can't rob sodium to pay zinc, can you?
Instead you have to make a new ion. And that's where zinc's former electrons come in.
Well, remember the electron from the zinc traveled down the wire to the copper electrode. So the copper now has the extra electron that balances out one of the sodium ions that lost its chloride, if we can put it like that.
But of course, the electrons can't build up on the copper as we mentioned above. If they do, the repulsion between the accumulating electrons will soon become so large that the electrons will stop flowing.
On the other hand, there's plenty of water at the surface of the copper. So for want of anything else to react with, the elections from the copper zap over to a water molecule.
2H2O + 2e- → 2OH- + H2
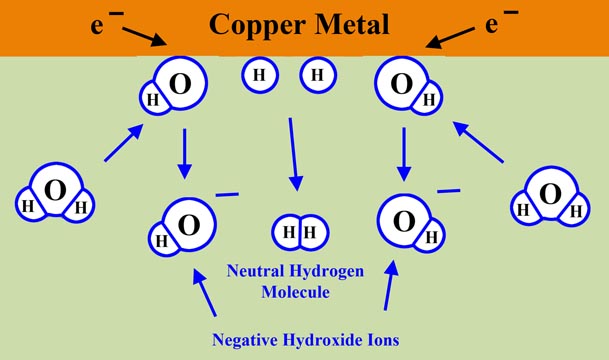
Water to Hydrogen and Hydroxide
So now the Zn+2 cation has an anion to balance its charge. That is, we now have zinc hydroxide.
Zn+2 + 2OH- → Zn(OH)2
We're showing zinc hydroxide as a single compound and not as the separate ions in solution because it's only slightly soluble in water and precipitates as a solid.
But note that when the water forms a hydroxide ion, there is a hydrogen atom produced. This links up with another hydrogen atom to form hydrogen gas.
H2O + e- → H. + OH-
2H. → H2
So in the voltaic pile - our early battery - the net reaction is:
Zn0 (metal) + 2H2O → Zn(OH)2 (aq) + H2
ΔGO = -19.5 Food Calories
A Gibbs Free Energy of -19 food calories is more than enough for a highly favorable reaction. So the reaction proceeds, and you get a working battery.
What we see, then, is that our battery does not create a true circuit of electrons - that is, no electron makes a complete circuit. It's more of a hand-off from the electrodes to the electrolyte and vice versa. But the point is that the electrons do move through the wires and produce useful electricity. And the bulb lights up.
Also notice that this battery actually chews up the zinc electrode and the brine ends up with zinc hydroxide - an insoluble material - gumming up the works. The fact that you have hydrogen bubbles forming at the copper also reduces the batteries performance. So eventually the battery runs down and in fact, the original voltaic piles were not that efficient or long lasting.
As you may expect the real world chemistry of the voltaic pile is more complicated than this basic explanation. For instance, it helps a lot if you add a bit of an acid to the electrolyte since the hydrogen atoms from an acid takes in an electron and produces hydrogen gas much easier than water alone. Also the copper electrode eventually corrodes although formally we see its function is simply catalytic.
But at least now you can say:
"I understand."
References
"The Periodic Table, and Why Batteries Don't Work the Way You Think", Davide Castelvecchi, Scientific American, October 13, 2011
"The Voltaic Pile: A Stimulating General Chemistry Experiment", Pirketta Scharlin and Rubin Battino, J. Chem. Ed., pp. 665-666, 1991.
"Off Goes the Power Current Started by Thomas Edison", Jennifer Lee, The New York Times, November 14, 2007.
