Robert Burns Woodward

The Greatest Synthetic Chemist?
Mebbe.
As a kid Robert Burns Woodward was such an exceptional student that he skipped grades three times. So he was only 16 when he enrolled at MIT where his passion was for the maturing field of organic synthesis.
However, precociousness has its problems. By the time Robert got to MIT he knew as much (if not more) organic chemistry than most advanced undergraduates. His professors quickly saw they had an exceptional student, and (albeit with some reluctance), they gave Robert bench space that was normally reserved for graduate students.
Robert liked the lab work. In fact, he liked it so much he began to skip classes. After all, he could learn the chemistry on his own. Naturally this didn't help his standing with the profs.
There's also that pesky little inconvenience that if you skip classes, you also skip taking and passing exams which, alas, is required to stay in school. Although the story doesn't appear to be true, one fairly authoritative biographical source says Robert was told not to bother returning for his sophomore year. Another story is that his grades dropped so far that he lost a scholarship and had to go to work. Still a third source says he almost dropped out but not quite. Whatever really happened, it seems, is one of the MIT professors, James Flack Norris, helped Robert work out a deal with the department. Robert did not have to attend classes as long as he showed up for (and passed) the exams. This he did with ease.
Since Robert had begun working in research from his first year, by the time he earned his bachelor's degree, it took him only another year to get his Ph. D. This was in 1937, and Robert was only twenty. That is, Robert had a Ph. D. before he could even vote! After a summer job at the University of Illinois (where he managed to tick off a few of the faculty), he became a research assistant at Harvard and then an instructor. Finally in 1944, Harvard promoted him to assistant professor.
Robert, like professors everywhere, was allowed to do consulting work. The Polaroid company had asked him to help them develop compounds that would be useful for their business. Robert was happy to do so. He did, after all, get paid above and beyond his professorial salary. But consulting and making compounds for a commercial firm, although it may be thirsty work, doesn't land you tenure. So Robert also asked Polaroid to fund some basic and fundamental research. Polaroid agreed, and Robert began working on the total synthesis of quinine.
Now a word of what a total synthesis is. Just synthesizing a compound does not a total synthesis make. You must start off with reasonably simple compounds which are routinely available. Then you proceed with a series of reactions where you know what will happen. Then if your reactions work, you end up with the compound you want, and you have a total synthesis.
So why do a total synthesis? The standard answer is that you now can make a rare natural product more easily. That is a laudable goal, yes, but in practice very few truly total syntheses are used commercially. Multistep syntheses are notorious for ending up with smidgens (and even microsmidgens) of the final product. If you begin with one gram of starting material and have 20 steps each with 90% yield, you'll end up with 0.01 gram - that's 100 milligrams - of your final product.
Instead, total syntheses traditionally have been much more important in proving the structures of molecules. Today, of course, structures are usually determined by fancy spectroscopic techniques - NMR, IR, Raman, mass spectroscopy, and x-ray diffraction. But these methods aren't perfect (even x-ray diffraction only works for crystalline solids). But in Robert's day many of these techniques were not routinely available. Instead, to prove a structure you started off with a nice simple compound whose structure was known and then performed a series of reactions where you know what will happen. Then if your reactions worked, you would end up with a compound whose structure you now knew.
In other words, you ran a total synthesis.
However, that still doesn't mean the overall goal of a total synthesis was only structure confirmation. If you did find a nice cheap way to make an industrially important or pharmaceutically valuable compound, tanto mejor. And generally people who funded that type of research hoped that is indeed what you would do. The valuable-compound-from-cheap-ones is also a convenient answer you could give to some in the - quote - "lay population" - unquote - (and even some fellow chemists) who sneeringly ask just what good is all that work you're doing.
Quinine, of course, has many uses. It was (and is) a flavoring for tonic water, and it has optical properties that make it useful in diagnostic tests. Commercially produced by extraction from the bark of the Cinchona tree, quinine was the only real effective treatment for malaria. And it was in short supply. After all, there was a war on - World War II - and malaria was a big problem with the troops.
Robert and his post-doctoral student (and later professorial colleague) William von Eggers Doering began the work to make synthetic quinine from a compound prepared from coal tar. It took about a year, and in 1944, they published a quick short "preliminary communication" in the Journal of the American Chemical Society. They expanded the article the next year to a full publication complete with experimental details.
Quinine from coal tar! It had been achieved! Even the great British chemist, William Henry Perkin the Elder, himself had tried to make quinine from coal tar, but only managed to make purple dye. Sure, that made William rich, but did he help suffering humanity?
Now with Robert and William's achievement, the news magazines could write that two young chemists had found the holy grail. Sure enough, Life Magazine - the #1 magazine in the United States - sent a reporter and photographer to Robert's lab, and Robert and William spent a week re-enacting the synthesis for the camera. On June 5, 1944, the magazine ran a layout complete with photos, one of which showed Robert smoking next to a reaction flask.
The article started out with a flourish:
Two 27-year-old chemists, Robert Burns Woodward and William von Eggers Doering, announced last month that they had made quinine by a laboratory process from synthetic chemicals derived from coal tar. This is the first time that quinine has ever been produced outside the life process of the tropical Chinchona tree, and the achievement is a climax of a long scientific search.
All well and good, mes amis. But what exactly did Robert and William do?
Their starting material was indeed a nice simple compound which was, as we said, derived from coal tar. This was 7-hydroxyisoquinoline:
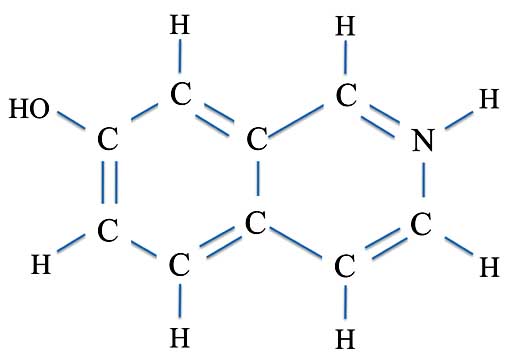
7-Hydroxyisoquinoline
Now here we must be honest. Although you might find some 7-hydroxyisoquinoline in coal tar, there isn't a lot. And it's not worth your while to try fishing enough out to start a long complex synthesis. So Robert and William made it in the lab from smaller molecules. Let us also caution the readers. Their méthode is not something to try on your own. In fact, following early synthetic procedures would often not be permitted by health and safety rules in your typical chemical laboratory today.
But whatever the actual starting materials, it was from 7-hydroxyisoquinoline that Robert and William had planned out an elegant and well-designed strategy. Briefly put (very briefly put), the route to quinine was:
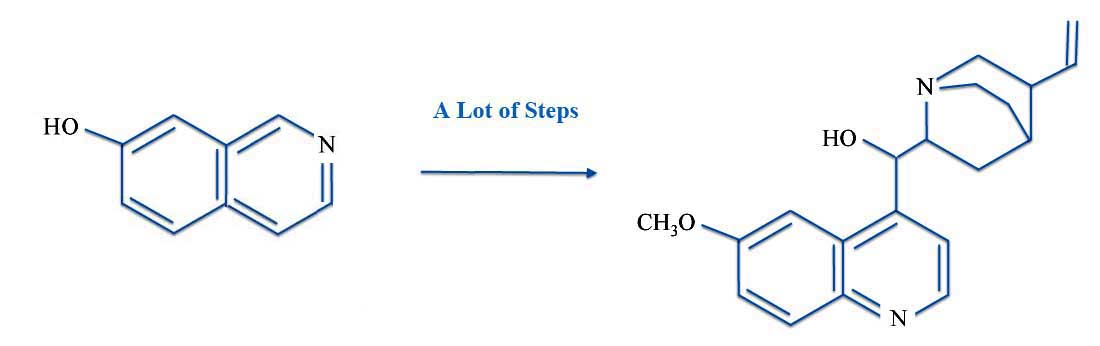
Total Synthesis: 7-Hydroxyisoquinoline to Quinine
Now the Life article was careful to point out - and the photos are really helpful - there was a crucial step of the synthesis. This was to prepare what Life called a "missing link", a molecule called quinotoxine which is a common (and shorter) name for 3-[3-ethenylpiperidin-4-yl]-1-(6-methoxyquinolin-4-yl)propan-1-one.
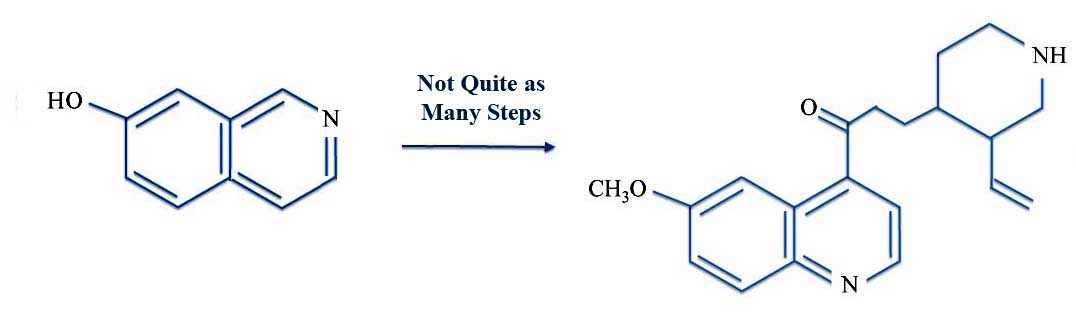
Almost to Quinine: 7-Hydroxyisoquinoline to Quinotoxine
Now just what did Life mean by dubbing quinotoxine the missing link?
Well, back in 1918 two German chemists, Paul Rabe and Karl Kindler, had shown that quinotoxine could be converted to quinine in three steps.
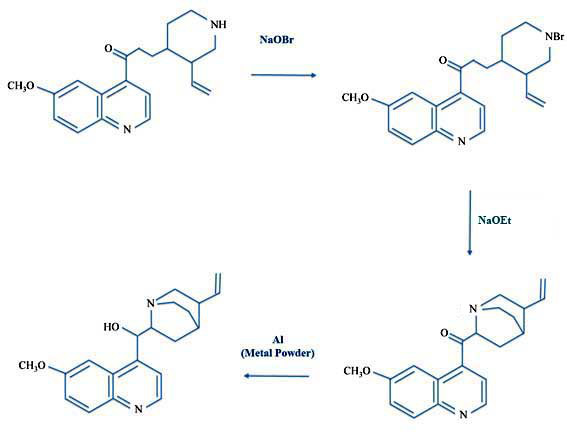
Paul and Karl: Quinotoxine to N-Bromo-Quinotoxine to Quininone to Quinine
So as long as Robert and William could make quinotoxine then quinine was a mere three steps away. So they made their quinotoxine and then Life reported Robert and William "thus assembled quinine, and all of its chemical cousins can be made."
But there's a wee bit of a problem with Life's grand finale.
First there is no guarantee you could make all of quinine's chemical cousins.
And besides, Robert and William did not make quinine.
They only made quinotoxine.
Or as they wrote in their own article:
In view of the established conversion of quinotoxine to quinine, with the synthesis of quinotoxine, the total synthesis of quinine was complete.
Hm. This does seem strange. In an article titled "The Total Synthesis of Quinine", Robert and William made no quinine but stopped three steps short. The rationale was - and the editor and referees of the article clearly agreed - that since Paul and Karl had already made quinine from quinotoxine, it was not necessary for Robert and William to do so.
Is this legit? Can you really make such a claim?
In the world of chemical synthesis, the answer is yes - at least to establish what is called a formal synthesis. A formal synthesis means that each step to make the compound has been demonstrated experimentally. On the other hand, no one chemist needs to run all of the reactions. So although we should call the synthesis the "Woodward-Doering-Rabe-Kinder" synthesis (actually some people like to add "Proštenik-Prelog" somewhere in the name), everyone now knew that synthetic quinine can be made.
Well, didn't they?
Uh - well - there's one little glitch.
It is a sad fact of life in the chemical laboratory that if you try to repeat what someone else has published, you often (some would say "mostly") do not get as good a yield as the author claims. And sometimes - perhaps more frequently than chemists like to admit - you don't get anything at all. The happy exceptions are the procedures published in the books titled Organic Synthesis.
And there is a perfectly straightforward reason for the reliability of Org. Syn reactions. Papers are not printed unless an independent researcher can verify both that the reaction works and that you get the reported yield. And despite the risk of calling down the ire of other chemical publishers and editors, the yields of Org. Syn are noticeably lower than those typically published in other chemical journals. Once one journal reported over 90 % yield for a product. And yet amusingly (at least if you're not trying to repeat the synthesis), when the same chemist wrote the synthesis up for Org. Syn, somehow the yield had mysteriously dropped to 60%.
OK. You usually get lower yields than reported and sometimes the damn reaction doesn't work. So what else is new? And what does this have to do with Robert, William, and quinine, anyway? What's the point?
Well, the point is that as long as the reaction of Paul and Karl really worked, then Robert and William were perfectly justified in claiming a formal synthesis of quinine. However, if Paul and Karl had made a mistake and their reactions didn't really produce quinine, then Robert's and William's much trumpeted claim was flat out wrong.
So we wonder. Is it reasonable to expect Robert and William to have least tried to repeat Paul and Karl's synthesis?
Well, it certainly seemed reasonable to a young graduate student at Wisconsin named Gilbert Stork. He even wrote to Robert and asked if he had tried Paul and Karl's reaction. As far as the records show, Robert never replied.
William later said he did not try Paul and Karl's reaction. And yes, you can advance some good reasons.
For one thing Paul and Karl provided pretty convincing analytical data that they ended up with quinine. They determined the molecular formula (C20H24N2O2, calculated by burning the compound and measuring the water and carbon dioxide), the melting point (177 oC, measured by melting the compound), and seeing how much a beam of polarized light rotated when you shine the light through a solution of quinine in ethyl alcohol. In all these tests, Paul and Karl's compound matched "authentic" quinine (that is, quinine extracted from the tree) perfectly. The odds they had something other than quinine was essentially nil.
Another reason not to bother with the last three steps was that Paul and Karl had provided no experimental details - that is, no temperatures, reaction times, weights of materials, or solvents. So it was sound reasoning just to accept the earlier synthesis as valid and declare a formal synthesis and not waste your time fumbling about.
But - there's a lot of "buts" in chemistry - the reactions Paul and Karl ran did not require any unusual procedures or materials. The first step was to take the quinotoxine and add sodium hypobromite (which is the same as laundry bleach where the chlorine is replaced by bromine). Next you take that product, N-bromoquinotoxine, and add what Paul and Karl called alkali (in this case, it was the strong base sodium ethoxide). The final transformation was a reduction - adding hydrogen atoms to the molecule - using aluminum metal in the presence of sodium ethoxide and ethanol. Again none of these reactions poses any particular problem to a synthetic organic chemist.
So why didn't Robert and William at least give the reactions a try? That would have silenced any Doubting Thomases (or in this case, a Doubting Gilbert) once and for all. What was three more steps in an already complex and lengthy multistep synthesis?
The obvious reason is that by the time Robert and William got to quinotoxine, they only had enough to prove they had quinotoxine, but not enough left over to run any more reactions. Now if you read about Robert and William's synthesis on the Fount of All Knowledge, some places say they got a 5 % overall yield. Now for a sixteen step reaction, that would be pretty good - an average of 83 % yield per step. But that yield doesn't gibe with the individual yields of each step reported in the full article. Tally those up - multiply each yield by the product of the previous yields - and you get - not 5 % but 0.5 %. Yes, one-half of one percent! Three more steps and Robert and William may very well have ended up with the proverbial microsmidgen.
But - and this is another big "but" - it is possible to make quinotoxine quite easily and in quantity by another route. After all, Paul and Karl had used it twenty-four years before Robert and William. So where did Paul and Karl's quinotoxine come from?
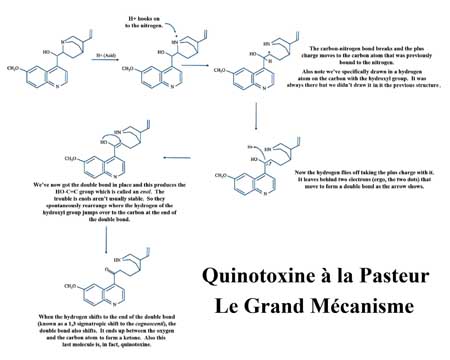
Chemists like to point out that Louis Pasteur - the same fellow who developed a rabies vaccine and learned how to pasteurize milk and wine - was actually a chemist. And in 1853 Louis found you could take "natural" quinine extracted from the tree bark and when you heat it with acid, you recover a quite good yield of quinotoxine. (Click on the image to the left and below to see Louis's reaction and the mechanism.)
Now Robert and William could easily have gotten hold of a pound of quinine which would have cost them about $12 (based on current prices corrected for the CPI). So they could have easily made enough quinotoxine to test Paul and Karl's reaction many times over. And if they had done so, their synthesis would no longer be just a formal synthesis. True, it would be what organic chemists call a "relay" synthesis, but a relay synthesis still counts as a complete total synthesis.
But as we said and for whatever reasons, William said he did not try Paul and Karl's reactions.
Well, maybe William didn't try the reaction. But what about Robert, arguably the greatest synthetic chemist of the twentieth century? Did he try the reaction?
Here we can confidently say absolutely not. William said it was he, he, William, who did the actual hands-on work. Robert - like all good bosses - stood around and watched.
In fact, as all bench chemists know, having the boss actually working in the lab is usually the last thing you want (in fact, you prefer them not to be in the lab at all). And in later years William - in good humor and with some diplomacy - said that laboratory work wasn't Robert's - ah- well - it just wasn't Robert's "strong point". In fact, William added, - not quite so diplomatically - that in the lab Robert was impossible. Oh, he was probably capable of going into the lab and boiling water. But if he had to boil an egg, William said, Robert would have problems.
You would think that other chemists eventually would at least try Paul and Karl's reaction. But for some reason they didn't.
What can we say? Did Robert and William even complete a formal synthesis of quinine? It looked like maybe not.
As chemistry moved into the twenty-first century, Gilbert, the graduate student who had written Robert and received (apparently) no answer, worked out his own synthesis that did end up with quinine. Gilbert was a bit critical that Robert and William had not even given the last three steps a try. By now Gilbert himself was an internationally recognized chemist and so his opinion carried a lot of clout. Soon some people - whom we will not mention by name - began talking about the "myth" that Robert and William synthesized quinine.
On the other hand, another chemist, Jeff Seeman, reviewed the historical data. His conclusion was that the original synthesis had indeed worked.
There is, of course, only one way to resolve the dispute. So in 2008 - sixty-four years after Robert and William appeared in Life - two chemists had another stab at the reaction. And they made a very interesting discovery.
The problem, it seems, lies with the quality of the aluminum in the last step.
The reaction just doesn't work if your aluminum is too pure.
Wait a minute. Don't you mean the reaction won't work if the aluminum is too impure?
Nope. We mean if the aluminum is too pure.
Why? Well, in 1918, aluminum was - to use modern chemical terminology - crud. It was kept in loosely stoppered bottles and if it had been sitting on the shelf long enough, it would have reacted with oxygen and ended up contaminated with aluminum oxide.
And what the later chemists found was that, sure enough, if you used fresh aluminum from the modern chemical supply house, the reaction fails. On the other hand, when they let oxygen react with the aluminum - that is you "aerate" the metal - you make small amounts of aluminum oxide. Then Paul and Karl's reaction works fine. You just need to use the same crud aluminum they did.
(The phenomenon of crud reagents working better than pure ones isn't unique to Paul and Karl's work. Once the Nobel Prize winning chemist Herbert Brown was going to run a reaction with zinc metal. Use "pure" zinc the (old) procedure stated. Try as he might, Herbert couldn't get the reaction to work. Then he went down to the basement storeroom and got the oldest, crudiest zinc he could find. He then worked up an excellent yield of his product.)
So the answer is, yes, Robert and William did indeed succeed in establishing the first total, albeit formal synthesis of quinine. This was one of the first multi-step synthesis of an important natural compound, and without doubt it was Robert's first big success. He began his (actually pretty quick) rise in the ranks at Harvard to full professor and for his synthetic research - which included synthesizing Vitamin B12 (using a relay strategy) - Robert won the Nobel Prize in 1965.
Ironically, Robert's most important legacy is not - though some may disagree - his synthetic work at all. What Robert did that was most important was when he teamed up with theoretical chemist, Roald Hoffman. Together they developed a set of simple rules for understanding why certain reactions proceed smoothly by heating and others required catalysts or photolysis (hitting the molecules with light). These "Woodward-Hoffman" rules made a big splash starting in 1965.
But even this research, too, brought on some issues of originality and attribution which we will not belabor here (you can read about it at various places on the Fount of All Knowledge). But when you get down to it, the foundation of the W-H rules really went back to work of the Japanese chemist, Kenichi Fukui, in 1952. Fortunately, Robert and Roald's work on what is called the conservation of orbital symmetry rekindled interest in Kenichi's simpler theory, and when Roald won the Nobel Prize in 1981, Kenichi did too.
In addition to having a prodigious memory and quick chemical intuition (necessary in the pre-computer era for synthetic chemists), Robert had - as should be obvious - an immense capacity for work. Chemistry was both job and hobby. But still, although Robert slept only three hours a night, he liked to enjoy himself. Almost everyone who knew him would relate Robert's fondness (and capacity) for daiquiris (sometimes drinking a pitcher during his three and four hour seminars), scotch (one chemist remembered Robert having three drinks before the first one), and a consumption of two to three packs of Benson and Hedges per diem. Robert died of a heart attack at age 62, on July 8, 1979.
References
"Quinine: Two young chemists end a search by making drug synthetically from coal tar", Life Magazine, June 5, 1944, p. 85.
Robert Burns Woodward : Architect and Artist in the World of Molecules, Otto Benfey, Peter Morris (Editors), Chemical Heritage Foundation, 2001. Largely reprints of Robert's most important papers but with a number of chapters by people who knew him, both personally and professionally.
"Robert Burns Woodward: Three Score Years and Then?", David Dolphin, Heterocycles, Vol. 7, No. 1, 1977.
"Robert Burns Woodward: 1917 - 1979", Elkan Blout, Biographical Memoirs, National Academy of Sciences, Volume 80, The National Academy Press, 2001.
"Robert Burns Woodward", Dictionary of World Biography, pp. 4502, Routledge, 1999.
"Robert Burns Woodward", Complete Dictionary of Scientific Biography, Charles Schribner's Sons, 2008.
"The Woodward-Doering/Rabe-Kindler Total Synthesis of Quinine: Setting the Record Straight", Jeffrey I. Seeman, Angewwante Chemie, International Edition, Vol. 46, pp 1378-1413, 2007. A detailed explanation of the quinine synthesis and the controversy it generated, but published before it was proven Paul and Karl's reaction really did work.
"Rabe Rest in Peace: Confirmation of the Rabe-Kindler Conversion of d-Quinotoxine Into Quinine: Experimental Affirmation of the Woodward-Doering Formal Total Synthesis of Quinine", Aaron Smith and Robert Williams, Angewandte Chemie International Edition, Volume 47, Issue 9, pp. 1736-1740, 2008.
"Quinine Synthesis Mystery Solved", Henry Nicholls, Chemistry World, Royal Society of Chemistry, http://www.rsc.org/chemistryworld/News/2008/February/05020801.asp.
"Über die partielle Synthese des Chinins. Zur Kenntnis der China-Alkaloide XIX", Paul Rabe and Karl Kindler, Berichte der deutschen chemischen Gesellschaft, Volume 51, Issue 1, pp 466-467, 1918.
Organic Synthesis, Collective Volumes,Wiley, 1941 to date.
Studies Toward the Total Synthesis of Quinine, Peter Andrew Webber, Ph. D. Thesis, University of Texas
Personal Communications from -----. Most of what you read here is based on information from the published papers, articles, and books. But a couple of places are personal reminisces of people who were there.